Please note that the prescription and issue of a wide range of medical devices by electronic prescription commenced on 1 November 2021.
The transitional period starting 1 May 2020 ended on 1 November 2021, which offered an opportunity for the electronic prescription and issue of medical devices in the pharmaceutical product ePrescription module of the National eHealth Infrastructure (EESZT), included only on the limited list of products authorised by the NHIFM. Thereafter, medical devices are prescribed and issued through the eGYSE module of the EESZT.
Please note that after launch of the separate electronic medical device (GYSE) prescription module, GYSE prescriptions issued earlier within the pharmaceutical product ePrescription module remain valid for an additional 3 months, until 30 January 2022!
The information hereunder summarises key information on the prescription and filling of eGYSE prescriptions.
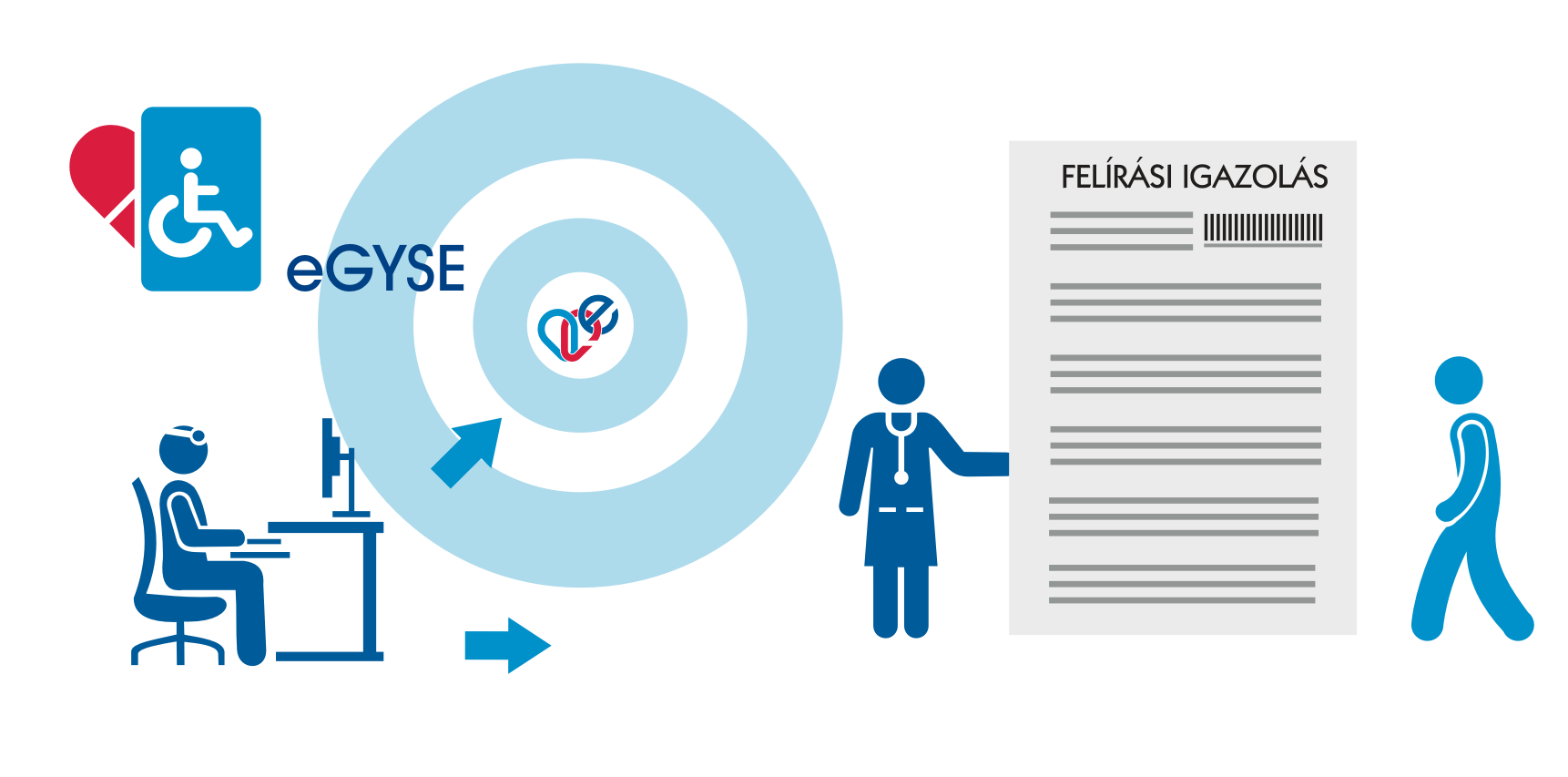
Similarly to the standard pharmaceutical product ePrescription, each physician writes the prescription with his/her own computer program, which automatically becomes an electronic prescription and is uploaded to the “Infrastructure”, from where it can then be queried from any medical device distributor and the given pharmacy in Hungary.
The earlier obligation of the prescribing party to issue a prescription certificate has changed as of 7 July 2021. For medical device and pharmaceutical product prescriptions by electronic prescription, the physician issues a prescription certificate only upon separate request of the patient (FIG). The prescription certificate is provided by the physician—at the patient’s discretion—on paper or in electronic form, hence the FIG can also be sent by e-mail to the patient as a pdf document.
Additionally, in relation to prescriptions for patients under the age of 14, the physician is no longer required to issue a paper prescription certificate for pharmaceutical product prescriptions uploaded to the EESZT and eGYSE prescriptions.
The prescription certificate can be printed on white A4 paper and can include data of several prescriptions. It is important that it is not necessary for the printed FIG to bear the physician’s signature and his/her stamp.

The issuer of the eGYSE prescription returns the paper prescription certificate to the person having it filled after expedition of the product (e.g. “Issued: day month 2021”).
The FIG containing an eGYSE prescription ID starting with 27 may also be filled on our own or someone else’s behalf.
Prescriptions can still be filled on our own or someone else’s behalf in the usual manner with a prescription certificate (pharmaceutical product ePrescription and eGYSE prescription) and in certain cases with traditional paper prescriptions.
Presentation of the prescription certificate is not compulsory in the GYSE store or pharmacy. Prescriptions with a prescription certificate can also be filled on the basis of the social security number.
Pursuant to Section 11/A of Decree No. 44/2004 (IV. 28.) of the Minister of Health, Social and Family Affairs, the physician may – ON AN EXCEPTIONAL BASIS! —issue a manual or software generated, paper-based prescription on the pink NHIFM prescription form if:
- issue of the eGYSE prescription is not possible (e.g. lacking EESZT or internet connection),
- issued for a medical bag,
- it is alternatively “seu”,
- for pharmaceutical products not marketed in Hungary,
- for “pro familia” prescriptions,
- the patient indicates that the pharmaceutical product will be filled abroad.
Prescriptions can be filled as follows without an eGYSE prescription identifier, on the basis of the social security number:

- by presentation of a personal ID card and TAJ card
OR

- with an e-ID card with a storage element also containing the social security number (TAJ), with entry of the PIN code.
For other persons:
By providing the social security (TAJ) number: providing the patient’s TAJ number together with identification of the person having the prescription filled.
According to the legislative amendment in force from 29 June 2021, if the electronic prescription is to be filled by provision of not on one’s own, but someone else’s TAJ number, the issuer is required to verify the personal identity of the person having the prescription filled, and to enter the ID of the presented document and the name of the person filling the prescription in the local system. The pharmacy or—for the issue of medical devices—the medical device distributor is required to process personal data recorded for issue for 5 years.
- Based on the authorisation registered in the EESZT: with personal identification of the authorised person registered in the EESZT, in possession of the patient’s TAJ number you can have the pharmaceutical products and medical devices prescribed for the represented person filled without requiring any other documents,
- Based on the legal right of representation registered in the EESZT: with personal identification of the legal representative registered in the EESZT, in possession of the patient’s TAJ number you can have prescriptions for the represented person filled without requiring any other documents.
According to legal regulations, the issuer of pharmaceutical products and medical devices issues a so-called issue certificate on the filling of electronic prescriptions. The person having the prescription filled acknowledges this by signing the issue certificate, with a device registering the signature or personal identification.
For the filling of prescriptions with an e-ID card, it is not necessary to print the issue certificate and to have it separately signed by the person having it filled. In such cases the PIN code substitutes the signature and the issue certificate is issued electronically.
If the EESZT is unavailable, the prescription may also be filled on the basis of the prescription certificate, where a paper-based issue certificate is issued in the medical device store or pharmacy. In such cases the person receiving the product acknowledges its receipt with his/her signature and by stating his/her official identification card number proving personal identity on the issue certificate.
Paper-based NHIFM prescriptions are entered into the EESZT at the time of their filling with the pre-printed prescription ID starting with 21 at the pharmacies and medical device distributors.
Similarly to electronic prescriptions for pharmaceutical products, the eGYSE prescriptions can also be queried and searched among Patient Documents on the EESZT Public Portal (eeszt.gov.hu).
New regulation is in force as of 1 November 2021 in relation to the delivery time and patient declarations . Decree No. 32/2021 (VII. 29.) of the Minister of Human Capacities amended Decree No. 14/2007 (III. 14.) of the Minister of Health on the acceptance of medical devices for social security funding eligibility, their ordering with subsidisation, distribution, repair and renting.
Pursuant to the legislative amendment, the patient—simultaneously with the issue of the prescription—declares in writing on the reverse side of the prescription that he/she has not had any prescriptions filled for medical devices with an identical purpose with social security financing up to the delivery time of the medical device.
“For electronic prescriptions, the patient issues the declaration writing, as part of the patient documentation, the availability of which is indicated by the physician in the EESZT”.
Thus, for eGYSE prescriptions , the patient declaration precluding parallel use of social security financed medical devices with an identical purpose must be made in writing, the fact of which is indicated by the physician in the EESZT when writing the prescription.
Please also note that during the emergency and for 90 days after its termination , the specialist medical recommendations will remain valid for pharmaceutical products and medical devices. The provisional extension of specialist medical recommendations must be recorded by the general practitioner in the health documentation of the patient.
The information document relating to medical devices available through electronic prescriptions is available here .
Additional information is provided under frequently asked questions and answers .
If you have any questions, please contact EESZT Key Account Helpdesk staff through the gyse.eeszt@okfo.gov.hu e-mail address or call the +36 1 920 1050 phone number.
Updated: 03.02.2022 10:51


